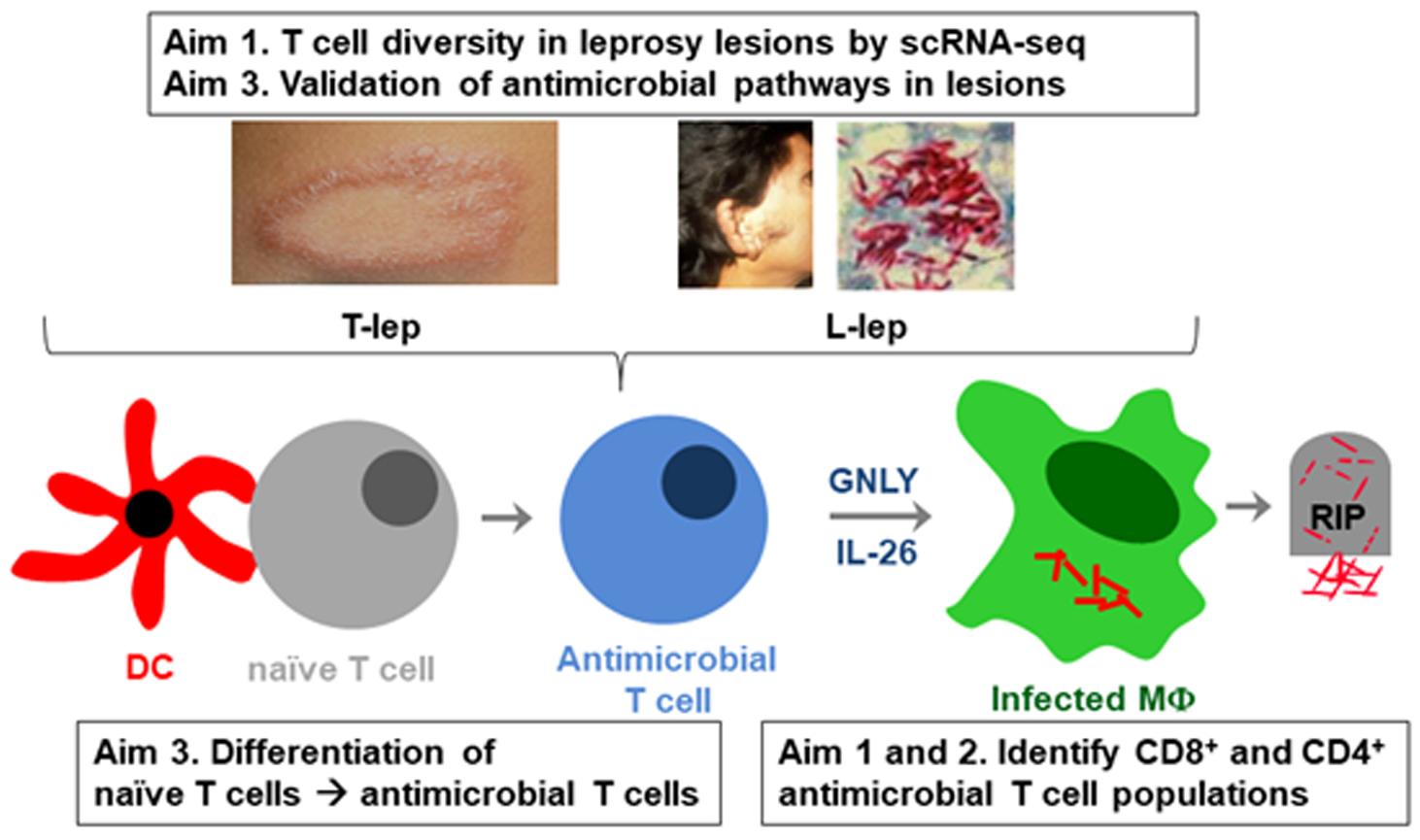
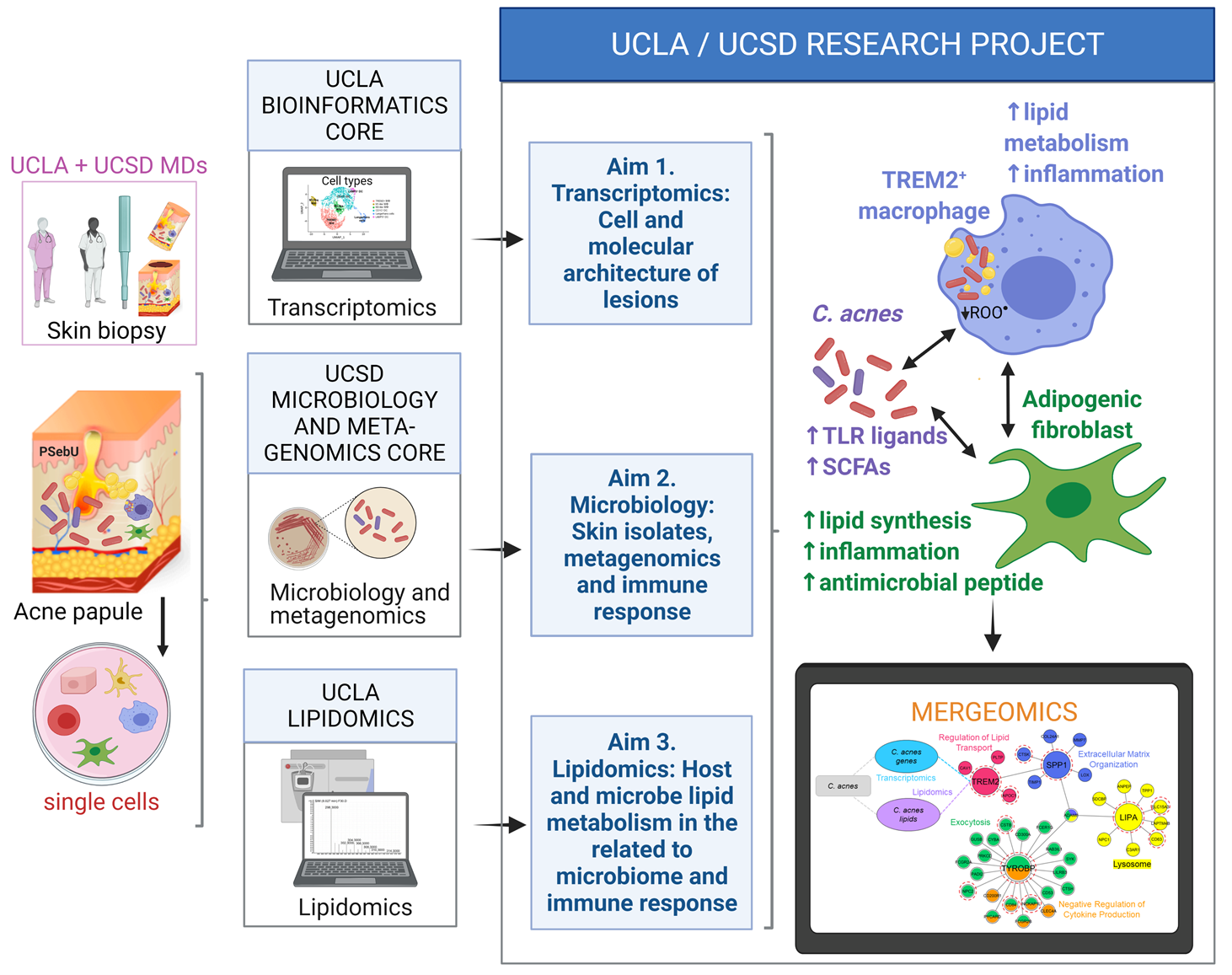
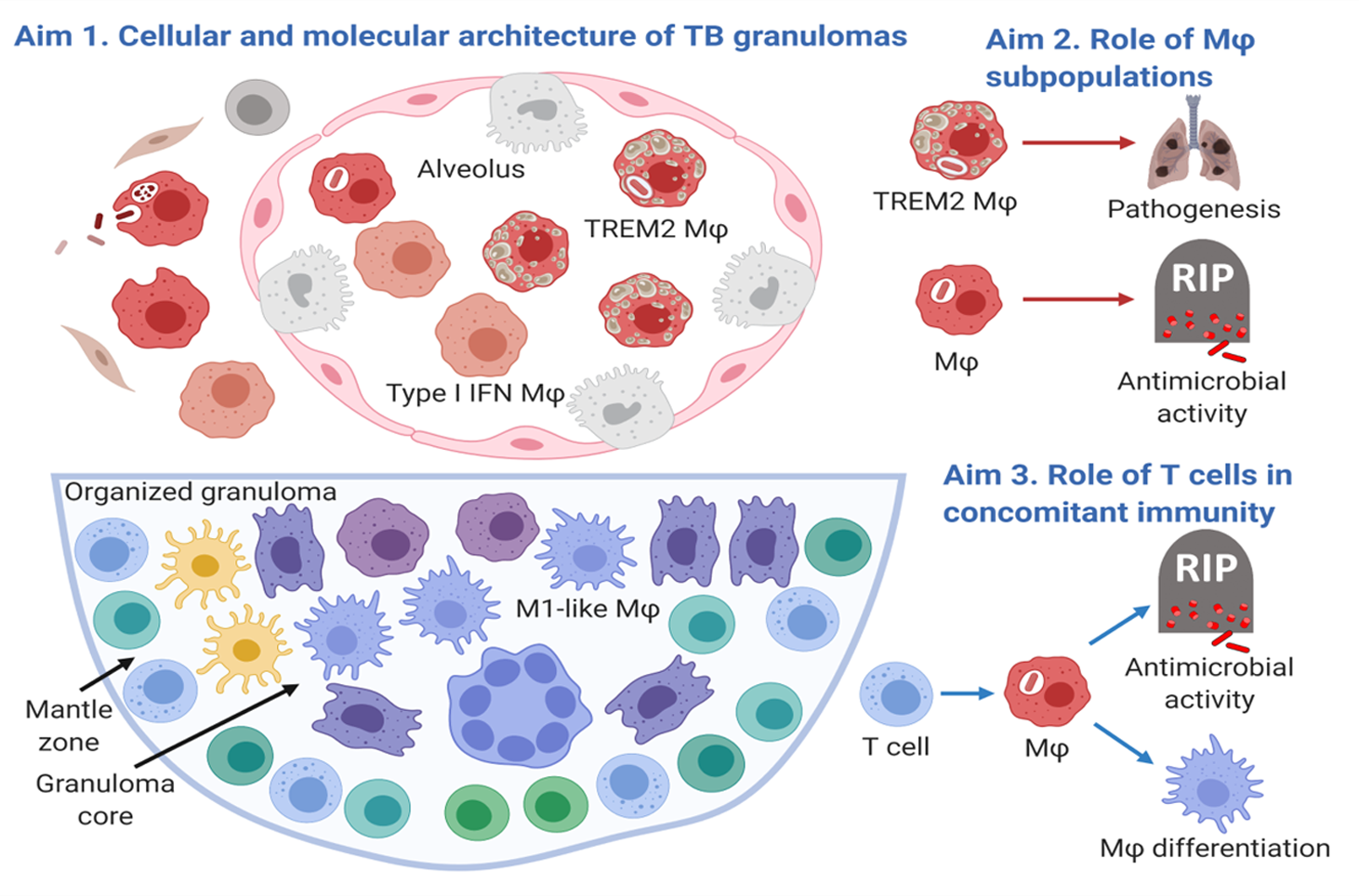
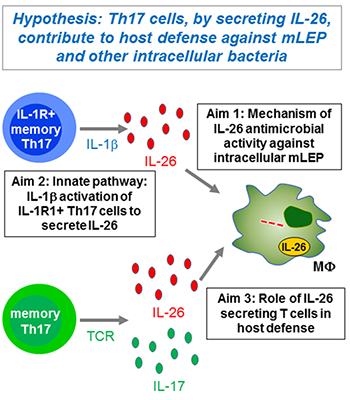
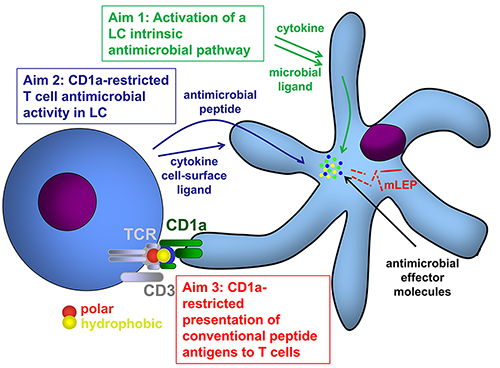
One crucial barrier mechanism via which the skin defends against microbial infection involves Langerhans cells (LCs), which are a skin-specific resident dendritic cell (DC) subset in the epidermis. Previous studies in our laboratory found that LCs contribute to the cutaneous immune response to Mycobacterium leprae, the causative agent of leprosy, via a langerin-dependent, CD1a-restricted antigen presentation pathway to T cells. We uncovered new paradigms of CD1 immunobiology, including defining the lipid-containing CD1-restricted T cell antigen structure and mechanisms of CD1 antigen presentation, mechanisms via which CD1 expression is regulated in skin, and the first evidence for direct T cell-mediated antimicrobial activity. To build on these findings, we aim to examine the role of CD1a-restricted T cell and LC in host defense in skin.
Potential projects:
-
Determine specific pathways by which activated LCs kill intracellular bacteria as well as microbial ligands and their role in LC-mediated antimicrobial response
-
Investigate mechanisms by which CD1a-restricted T cells kill intracellular pathogens in LCs
-
Define mechanisms by which LCs process and present CD1a-restricted bacterial peptides to T cell