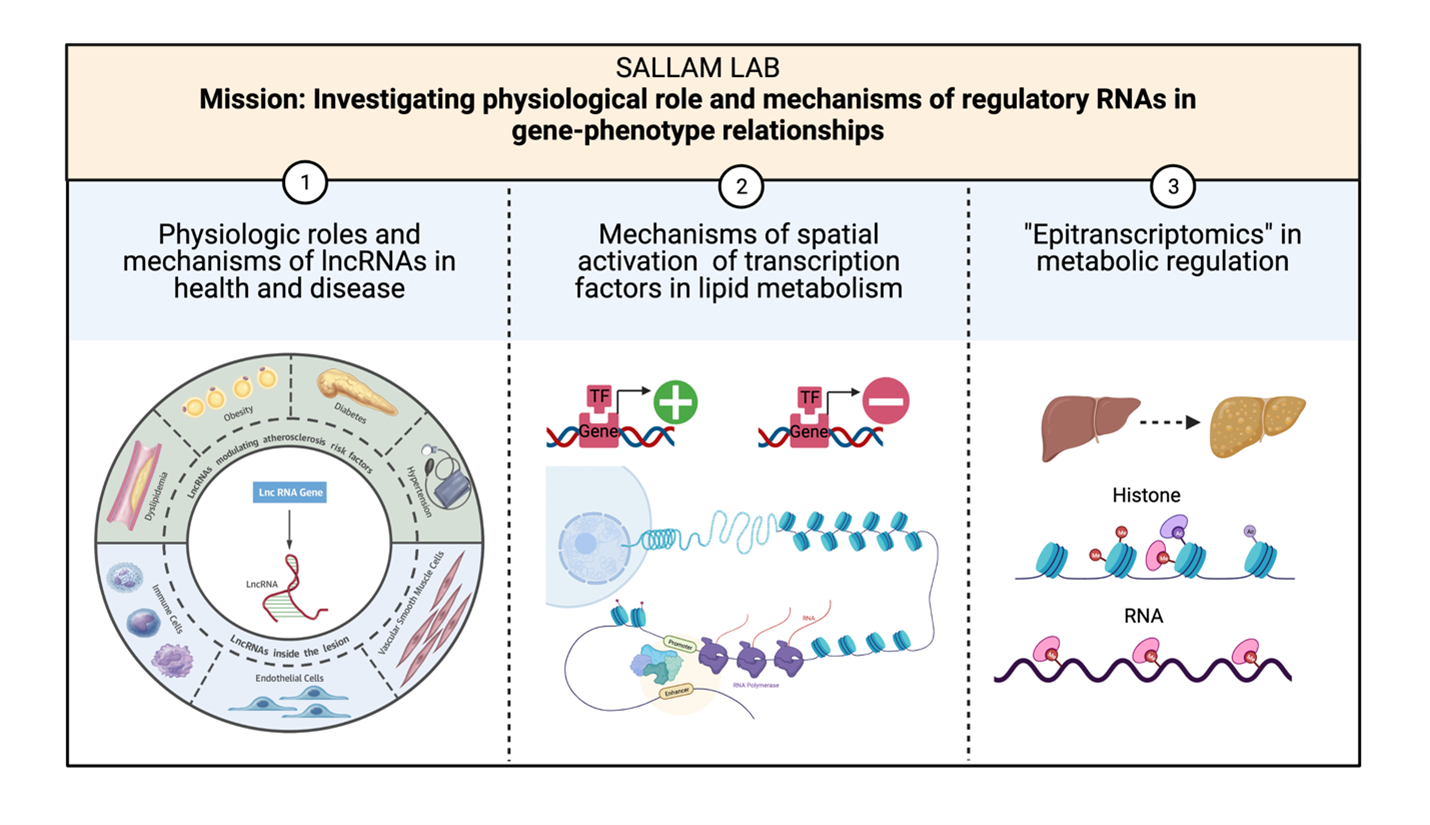
Our Mission: How can a 30-year-old with no cardiovascular risk factors develop a deadly heart condition? Reciprocally, can a patient with a heavy risk factor burden develop resistance to plaque build-up? Inspired by common real-world mysteries and taking a “gene regulation centric” approach, our lab investigates the physiology and mechanisms of Regulatory RNAs in cardiometabolic diseases. Our efforts focus on several key areas:
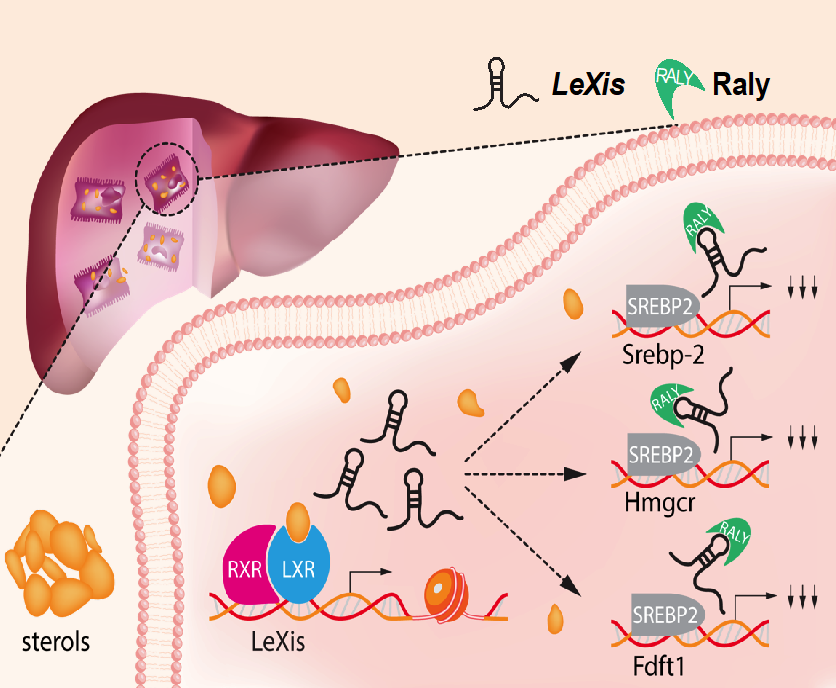
Contribution of noncoding RNAs and their binding partners in metabolism and atherosclerosis.
Long noncoding RNAs are arbitrarily defined as noncoding transcripts greater than 200 base pairs. Multiple lines of evidence over the last decade provide significant knowledge about lncRNAs functional roles, molecular interactions, and relevance to diverse human diseases. Utilizing molecular, cell biological and human disease models, our lab investigates the physiologic role and mechanisms of lncRNAs in cardiometabolic health and disease.
Epigenetic and transcriptional control mechanisms in metabolism. Regulation of gene expression by metabolic transcription factors is remarkably “temperamental”. Despite minimal variation in their binding patterns, outputs of nutrient-sensing transcriptional factors (TFs) can vary substantially depending on context. Using unbiased chromatin interrogation approaches our group dissects cooperative relationships of TFs impacting genes critically involved in 1) hepatic metabolic control and 2) macrophage responses in atherosclerosis.
Epitranscriptomics in metabolic regulation: Our group has discovered that chemical modifications on RNA are essential for precise control of hepatic responses to dietary cues. In addition, RNA modifications may contribute to sex differences in metabolic traits. We interested in investigating the mechanisms by which RNA modifications drive sex-dimorphic traits and mechanisms of crosstalk with epigenetic circuits.
_(1).gif)
Genetic basis of extreme lipid traits. An emerging area of interest in our lab is investigating the contribution of novel gene perturbations in patients with highly unusual lipid abnormalities. Utilizing multidisciplinary approaches including novel knockout or gain of functional models these studies leverage our unique strengths at UCLA boasting one of the largest and long-standing Lipid Specialty Centers in the world serving our diverse patient population.
We are currently hiring. For further information please contact: tsallam@mednet.ucla.edu