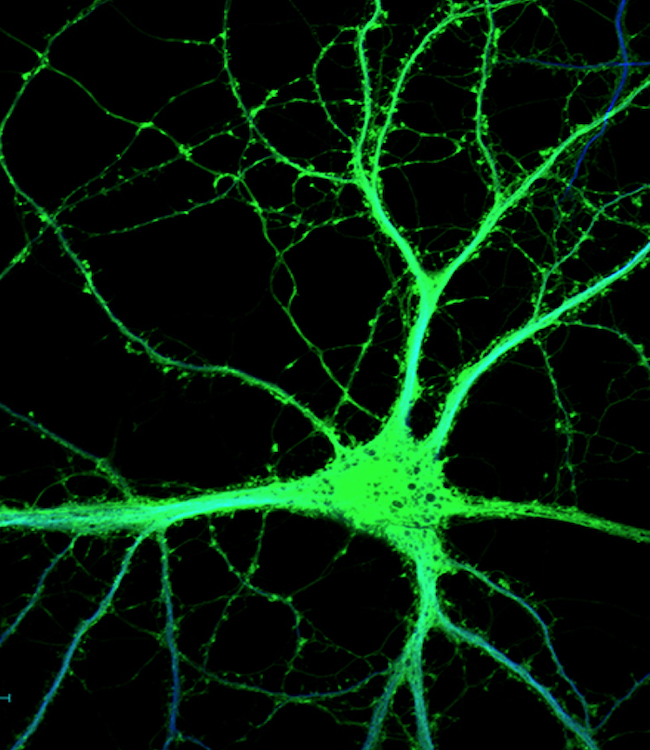
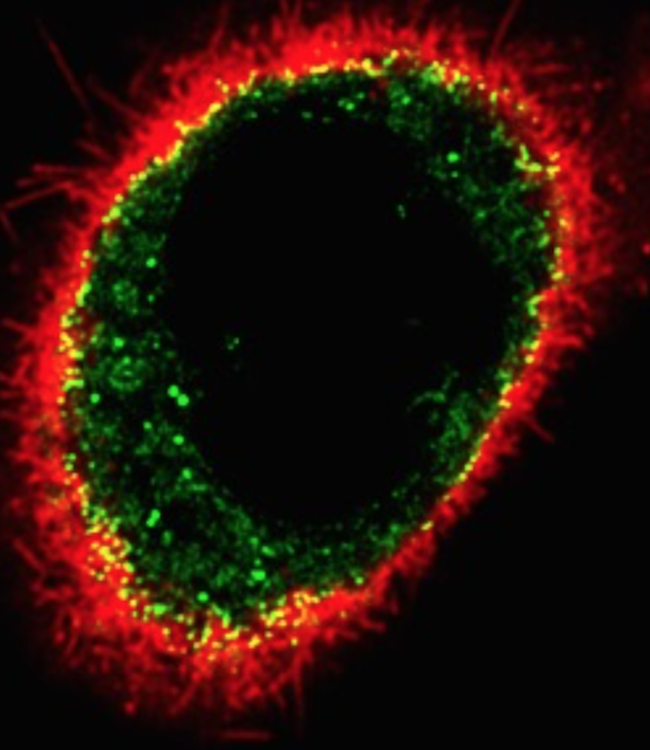
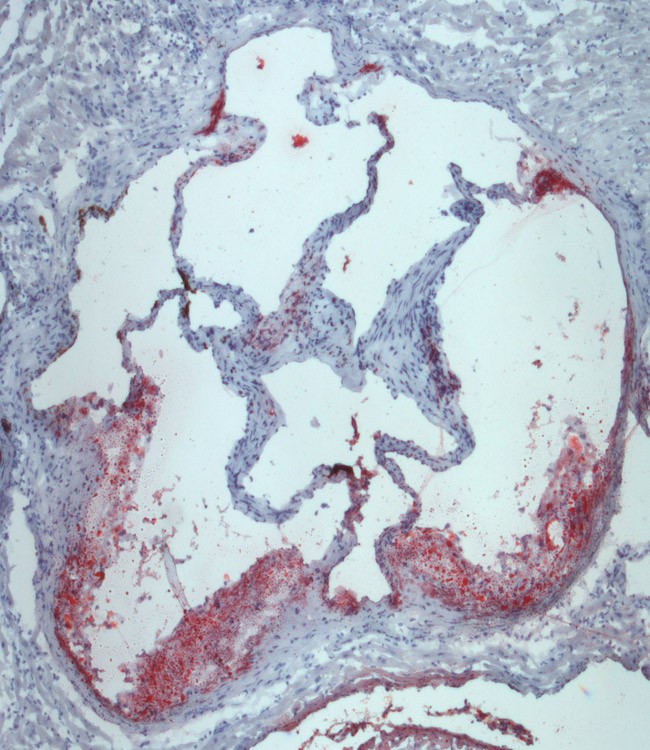
The Tontonoz Laboratory is in the Department of Pathology and Laboratory Medicine in the David Geffen School of Medicine at UCLA. The laboratory is located at the MacDonald Research Laboratory (MRL).
Principal Investigator
Peter Tontonoz, MD, PhD
University of California, Los Angeles
675 Charles Young Dr., MRL 6-770
Los Angeles, CA 90095-1732
Office (310) 206-4546
Lab (310) 206-4622
ptontonoz@mednet.ucla.edu
Administrative Assistant:
Ingrid Gonzalez
(310) 825-9744
IDGonzalez@mednet.ucla.edu
Lab Manager:
Jon Salazar
(310) 206-4622
JVSalazar@mednet.ucla.edu
Latest News Articles
High-Cholesterol Diet Causes Tumours to Form 100 Times Faster: Discovery of how fatty substance drives cancer growth could pave the way for future drug treatments
Newly Discovered Gene May Protect Against Heart Disease
Latest Publications
IDOL regulates systemic energy balance through control of neuronal VLDLR expression
Obesity, diabetes and cardiovascular disease are the leading causes of morbidity and mortality in industrialized societies. The common thread that links these disorders is dysregulation of lipid metabolism. The focus of the Tontonoz laboratory is the regulation of the regulation of lipid metabolism and the links between lipids, inflammation and immunity, and metabolic diseases.
Work in our group over the past nineteen years has revealed that the lipid-activated nuclear receptors PPAR and LXR function as coordinators of lipid metabolism and modulators of immunity and inflammation. These transcription factors positively regulate the transcription of a battery of genes directly linked to lipid uptake, efflux and transport, and negatively regulate genes involved in inflammatory responses. Our studies have revealed new mechanisms whereby cholesterol and triglyceride metabolism impact the functions of immune cells. In addition, we have provided in vivo evidence that the LXR and PPAR signaling pathway are important determinant of atherosclerosis, insulin resistance and immune tolerance.
Ongoing work is centered on novel downstream mechanisms by which LXR, PPARs and their target genes regulate lipid and immune homeostasis. We have identified and characterized novel players in the control of lipid homeostasis, including the E3 ligase IDOL, the lncRNAs LeXis and MeXis, the phospholipid remodeling enzyme Lpcat3 and the Aster family of sterol transport proteins. Future studies are expected to provide insight into fundamental regulatory strategies and may identify targets for therapeutic intervention in human metabolic disease.