The Wanner team combines imaging and biochemical approaches at the intersection of structural and molecular neuroscience with direct translational use in the clinic.
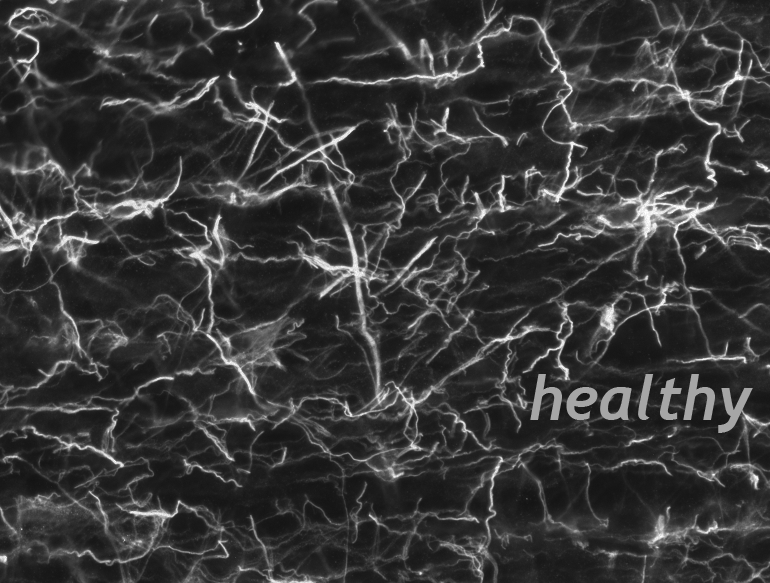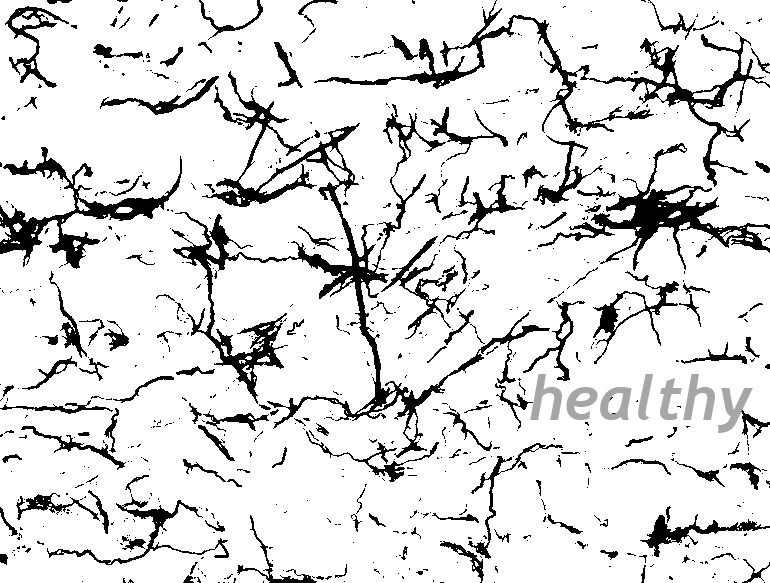
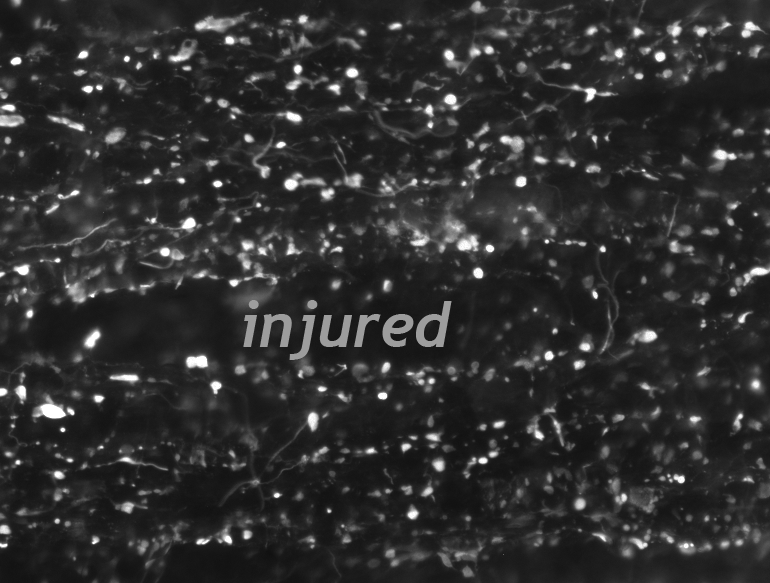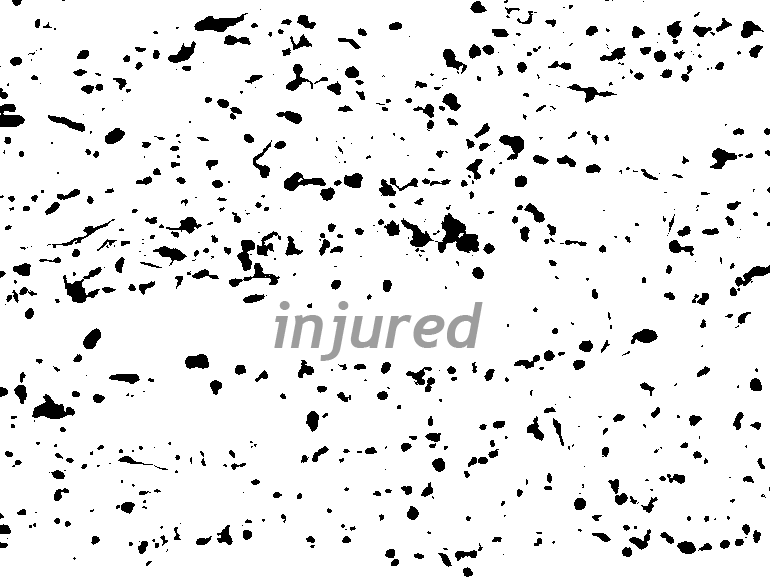
The Wanner lab established creative microscopic imaging tools and quantitative analyses using confocal microscopy, time-lapse imaging and ultrastructural studies (Publications). We measure biomarker changes in single cells, quantify GFAP filament aggregation, local protein translation with subcellular resolution and determine the extent of cellular injury including astrocyte fiber beading, called clasmatodendrosis, and cell body swelling, called cytotoxic edema after trauma.
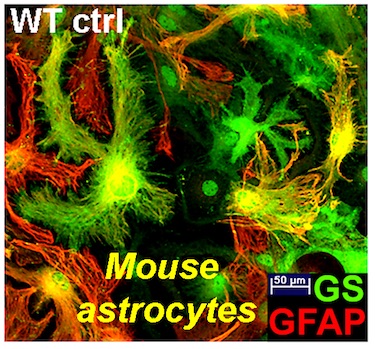
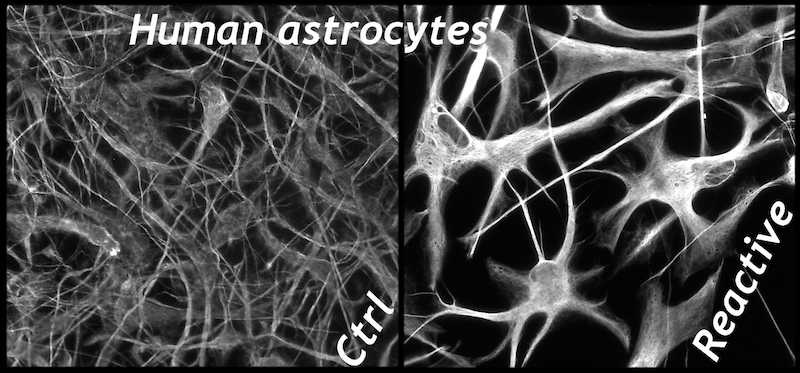
Dr. Wanner developed a novel human trauma culture model that recapitulates key aspects of cellular wounding and injury mechnisms to mechanical shear and deformation forces using abrupt pressure pulse stretching of cells grown on deformable membranes (Publications). Cortical rat and mouse or human astrocytes are traumatized for studying injury mechanisms and signaling. Neocortical human astrocytes are differentiated in serum-free medium and are well characterized by known and new astroglial markers and morphologies. Human astrocytes display exceptional heterogeneity and survival features (Publications). Rat, mouse and human neuron-glial co-cultures are established to address distinct questions of signaling and trans-differentiation between axons of cortical, spinal or dorsal root ganglion neurons and glial cells such as Schwann cells or astrocytes (Publications). Distinct stages of glial cells are studied in their inhibitory or supportive roles for axon outgrowth, which is needed for successful regeneration after spinal cord injury.
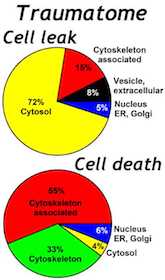
The labs of Drs. Wanner and Loo and the UCLA Molecular Instrumentation Center (MIC) worked jointly on the proteomic biofluid signatures after traumatic injury to human astrocytes, to the human brain and to the swine spinal cord (http://loolab.chem.ucla.edu/index.html). We established quantitative proteomic approaches including parallel and multiple reaction monitoring (PRM, MRM) and tandem mass tag TMT proteomics to measure protein changes caused by trauma.
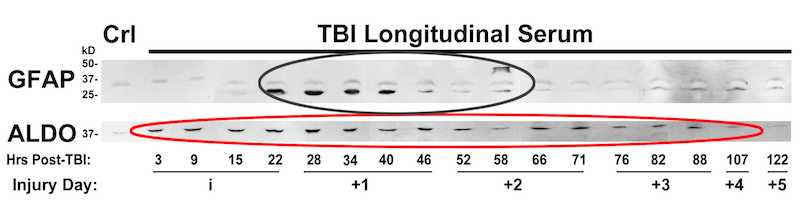
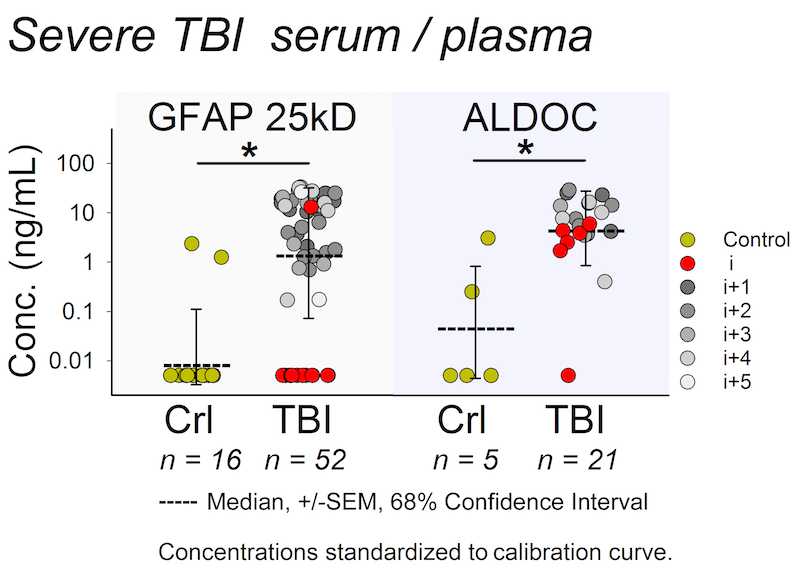
Biomarker released after trauma shows a wide dynamic range. The Wanner lab established quantitative densitometry on culture and clinical biofluids capable to measure non-saturated chemiluminescence over five orders of magnitude using cross-exposure scaling (Halford, Chong, Shen and Wanner, manuscript in preparation).